A Shot of Science
Vaccines are among the most significant achievements in public health. Since 1924, childhood vaccinations have prevented more than 100 million cases of serious disease.
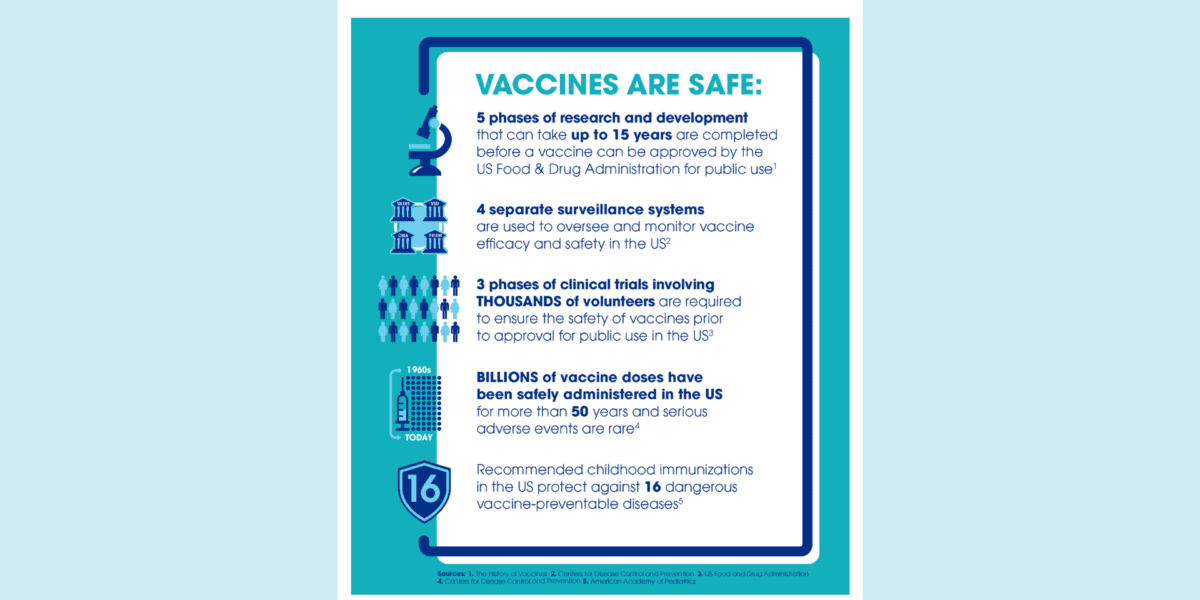
All vaccines used in the US are required to go through extensive safety testing before they are licensed by the US Food and Drug Administration (FDA) or recommended for widespread use.
After a vaccine is licensed, FDA and the Centers for Disease Control and Prevention (CDC) work with healthcare professionals to monitor the safety of vaccines, including any adverse events, especially rare events not identified in pre-licensure study trials. There are four systems in place in the US to monitor the safety of vaccines after they are licensed and used among the public, and new systems have been established to help monitor vaccines for COVID-19. These systems can monitor known side effects and detect rare side effects that may not have been identified during clinical trials.
One of the systems used to monitor the post-licensure safety of vaccines in the US is the Vaccine Adverse Event Reporting System (VAERS). VAERS accepts reports from healthcare professionals, vaccine manufacturers, and the general public and receives more than 25,000 reports per year, compared with millions of vaccine doses administered.
#ShotOfScience Campaign
NFID developed a #ShotOfScience campaign to share tools and resources on the history and science of vaccines. Campaign materials include sample social media posts and animated graphics.
A Shot of Science: Brief History of Vaccine Accomplishments
Infographic detailing the history and timeline of vaccine development
For more information about how vaccines are developed, approved, manufactured, and added to the US Recommended Immunization Schedule, view the CDC infographic, The Journey of Your Child’s Vaccine.
The National Academies of Sciences, Engineering, and Medicine has also compiled extensive information about vaccine safety.
Updated May 2022
Sources: Centers for Disease Control and Prevention, Department of Health and Human Services, National Academies of Sciences, Engineering, and Medicine
Related Resources
Building Vaccine Confidence Among Patients and Parents with Patricia (Patsy) A. Stinchfield, RN, MS, CPNP
S1, E2: NFID President Patricia (Patsy) A. Stinchfield, RN, MS, CPNP, shares her insights on storytelling and effectively responding to parent and patient concerns about vaccines …
NFID Statement on COVID-19 Vaccine Safety
FOR IMMEDIATE RELEASE Bethesda, MD (April 13, 2021)—The following statement can be attributed to William Schaffner, MD, medical director of the National Foundation for Infectious…